If you chop up a piece of semiconductor crystal into tiny pieces, it will develop some interesting properties. When you get to chunks of a few nanometres – 10,000 times thinner than a hair – they become nothing like the bulk material you started with.
Optical and electronic behaviour dramatically change with size. Nano-sized semiconductor crystals behave like zero-dimensional point objects, and they go by the name of quantum dots (QDs). They can be very efficient emitters of light with very high colour purity, and have already been used to provide the bright colours of television displays. Those QDs emit in the visible spectrum. But my work focuses on the invisible, infra-red part of the spectrum, which is more relevant for solar power applications.
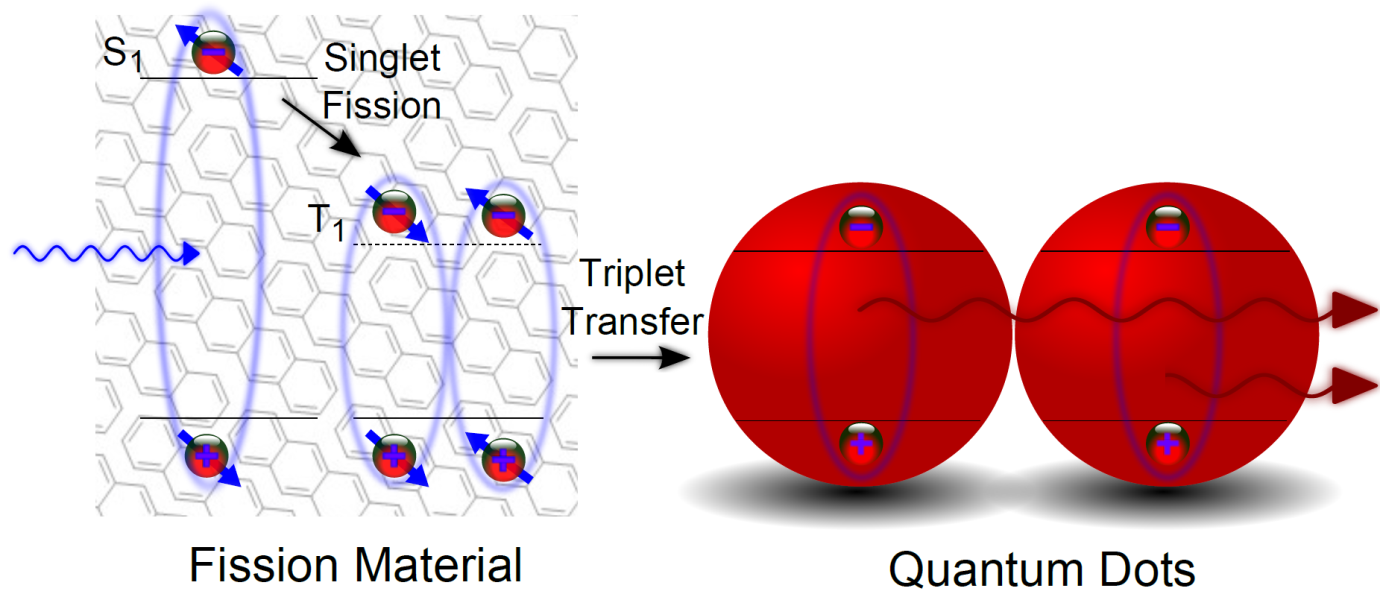
In the Optoelectronics Group at the Cavendish, we have been working on a system to convert high-energy blue photons from sunlight into double the number of low-energy infra-red photons (this is referred to as “down-conversion”). Infra-red is absorbed much better by silicon solar cells, whereas only a portion of blue light is absorbed with the excess wasted. By converting one high energy photon into two low energy and easily absorbed ones, more current can be obtained from the same area, leading to greater efficiency.
So far, this system has demonstrated excellent efficiency in two of the three key processes required., singlet-fission and triplet transfer. The third process, the key limiting factor is the emission efficiency of infra-red QDs, which are not as good as those QDs in the visible spectrum. My research is focused on improving the emission efficiency of these QDs and understanding the structure-property relationships that lead to high emission nanocrystals. Typically, nanocrystals are made brighter by encapsulating them with a nm-thick shell of a different material, but this messes up the second of the three key processes, the triplet transfer. The solution thus lies by striking a fine balance between different actions. In my case these are the nanocrystal formation and the shell growth. Only when this is achieved, luminous infra-red QDs can be achieved that can then be used to increase the efficiency of solar cells.
James Xiao
NanoDTC PhD Cohort 2014
Optoelectronics Group, Physics
Wright Group, Chemistry