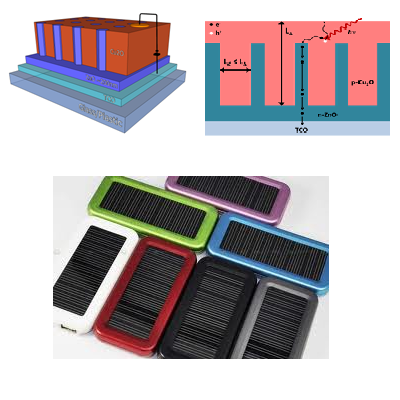
Powering everyday technology with solar cells
Sunlight is the most abundant source of energy on the planet. Photovoltaics – the science behind turning sunlight directly into electricity – has been studied for over 70 years and the result is the now familiar solar panels we see on roofs and in new ‘solar farms’ everywhere around the world. Our research goes one step further: Imagine if all the gadgets we use every day, from home appliances to mobile phones, never needed to be plugged in again. We design solar cells that are cheap and robust enough to escape our relying on grid electricity.

I use nanostructured metal oxides to achieve this. I look specifically at two materials: Cuprous oxide (Cu2O) and Zinc oxide (ZnO) which can be combined to make solar energy conversion devices or ‘cells’. Our cells have the advantage of being:
- Cheaper than standard silicon used for solar panels
- More durable than other low-cost materials like organic photovoltaics
- Capable of competitive energy efficiencies (20% theoretical, ~10% realistic)
Cu2O|ZnO diodes are capable of delivering ultra-low cost cells with target power conversion efficiencies of around 5-10%, comparable with commercially available silicon cells but at much lower cost. My PhD project focuses on using electrodeposition of Cu2O and hydrothermal growth of ZnO nanowires to improve Cu2O|ZnO diode formation and photocurrent density.


Introducing a nanowire architecture in ZnO (Fig. 2) allows a higher fraction of electric charge (electrons and holes) created by photons of light to be successfully extracted from the cell without falling back into lower energy states where the charge is immobile and stays in the cell. Electrodeposition allows Cu2O to be coated on ZnO nanowires conformally, giving the best chance of a well formed diode interface between the two oxide semiconductors. This creates a diode cell in which Cu2O absorbs light and ZnO extracts electrons from the cell, but with the nanowire structure shortening the path between where photon absorption and electron extraction occur (Fig. 3).
I am also looking for collaborators who explore devices from bottom-up growth of nanowires or semi-transparent p-type semiconductors as well as anyone with expertise on theory or characterisation of electrical behaviour associated with interface defects.