Nanoparticle catalysis is used in every day, such as in catalytic converters, or large-scale industrial processes which for instance aim to connect carbon atoms together. The point of catalysis is to reduce the energy requirements of a reaction to make it faster and less expensive. The use of nanoparticle catalysts is beneficial due to the high surface area of these materials, which is where the reactions take place. Furthermore core-shell nanoparticles with different elements on the inside and outside, have demonstrated the ability to reduce the amount of expensive metal catalysts required to catalyse a reaction. For example, platinum-coated silver nanoparticles are more catalytically-active than just metallic platinum nanoparticles for the production of hydrogen from formic acid. This makes the process less expensive and also brings about an interesting question: why is a platinum more catalytically-active for this reaction when it sits on top of silver?
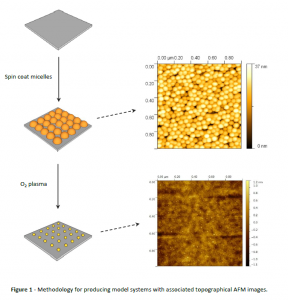
My PhD concerns the use of bimetallic nanoparticles for catalytic purposes, ranging from hydrogen production to the synthesis of carbon nanotubes. Thus far, I have demonstrated the templating of nanoparticles using the block copolymer PS-b-P4VP (20k-b-20k) to produce model systems that allow fair comparison between nanostructured samples in the 2-5 nm range. I have also used colloidal methods to synthesise a range of nanoparticles for various potential applications. The figure below demonstrates nanoparticle templating using PS-b-P4VP on a silica substrate.

Nanoparticles can be analysed by a range of techniques that, when used together, provide evidence for the types of atoms contained in the nanoparticle and their arrangement. One of the most important techniques is high-resolution transmission electron microscopy (HRTEM) which allows us to identify whether a nanoparticle contains more than one metal. Since our investigation aims at bimetallic nanoparticles, we need to be certain that the nanoparticles we have made individually contain both metals, so this technique is crucial. X-ray diffraction (XRD) can provide supporting evidence that two metals are present within the same nanoparticle, but this technique works best for larger particles that are > 10 nm, whereas our nanoparticles are often sub-5 nm. Other techniques for analysing colloidal nanoparticles include dynamic light scattering (DLS), inductively-coupled plasma-mass spectrometry (ICP-MS) [PG1] and UV-Vis absorption spectroscopy.
Analysis of HRTEM images by size distribution and EDX is shown below:

We aim to consolidate different aspects of the project to thoroughly investigate bimetallic nanoparticles, looking mainly at the effect of core-shell morphologies on catalytic activity. Going beyond the current advances in core-shell nanoparticle catalysts, we are focussing on hydrogen production from methane, ammonia-borane complexes and methanol, for which this morphology is very interesting as a potential method to increase catalytic efficiency.
As well as implementing colloidal techniques, we use triblock copolymers to template core-shell structures in bimetallic nanoparticles, with a view to producing core-shell structures below 5-nm. This is a highly-controlled method which allows for greater reproducibility and reliability in results.Adam FraserNanoDTC PhD Student Cohort 2010