Did you know that 150 million tons of plastic products are thrown away every year? This represents not only a severe environmental hazard but also a waste of valuable resources. Turning rubbish into hydrogen could be a solution.
You may have heard hydrogen (H2) endorsed as a green energy source. However, 96% of all H2 is produced by breaking down fossil fuels with steam, a process that not only requires non-renewable feedstocks but also consumes large amounts of energy and releases greenhouse gases.
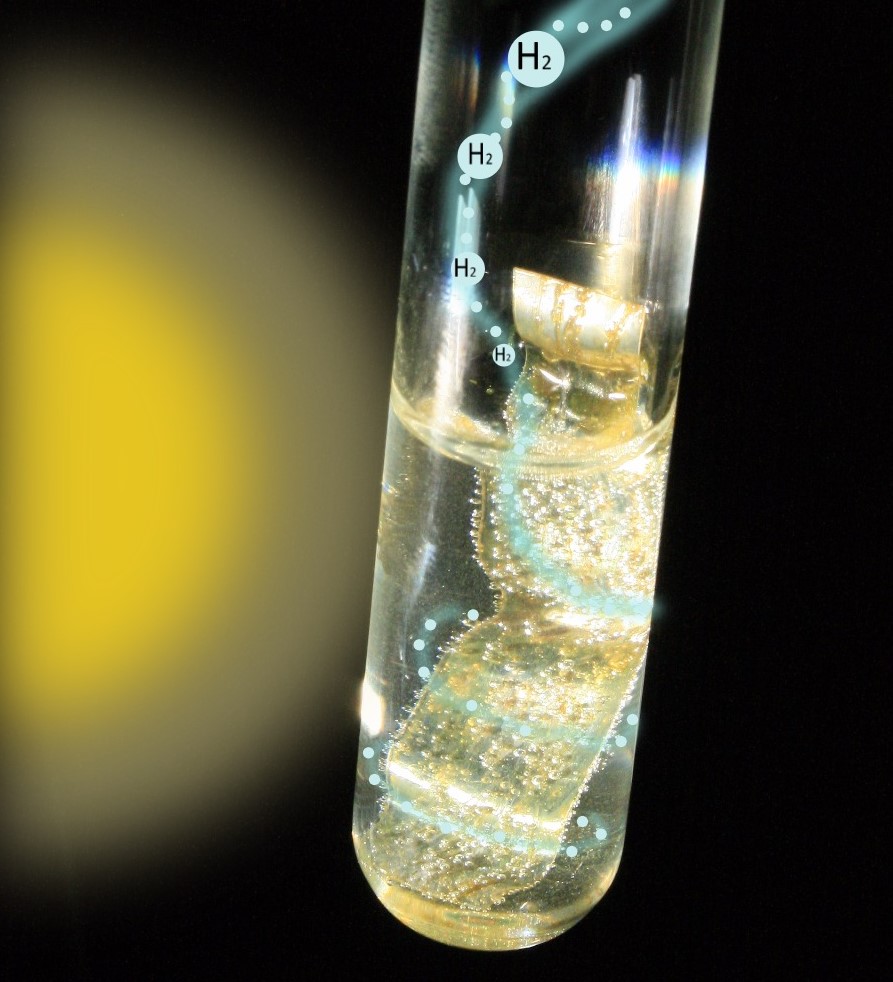
My research focuses on addressing these two issues – waste accumulation and non-renewable H2 production – with a catalytic method known as photoreforming. Photoreforming requires four “ingredients” to make H2: a photocatalyst, substrate, sunlight and water. A photocatalyst is simply a material that uses the energy in sunlight to make a reaction happen faster. In my case, the photocatalyst simultaneously breaks down the water and substrate (which can be various types of waste, from wood and grass to plastic water bottles and insulation foam) to form H2 and other small useful chemicals. It is a simple one-pot process that does not produce carbon dioxide and only uses the energy in sunlight.
As an emerging technology, photoreforming still has some limitations. These include the use of either toxic or expensive photocatalysts, long reaction times, and low H2 yields from certain substrates. Yet these challenges are precisely what make this process such an interesting topic of study. In my PhD, I aim to further develop our understanding of photoreforming by (a) testing additional types of waste and characterising the waste breakdown (oxidation) reactions, (b) identifying alternative photocatalysts, (c) up-scaling the process in order to prove its real world viability, and (d) conducting a life cycle assessment of the system. Eventually, I hope to prove that photoreforming can help turn those 150 million tons of plastic waste into useful products in a renewable and economic way.
NanoDTC PhD Student 2016