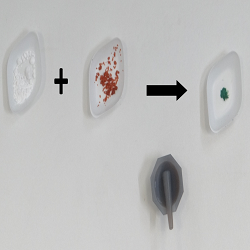
Mechanical grinding drives chemical reactions
Typically, chemists do reactions by dissolving their starting materials and then heating the solution. An alternative is to use brute force. Grinding solid chemicals together can induce reactions without needing toxic solvents or heat so the environmental cost is far lower than for traditional means. Grinding reactions have been used to make a wide variety of materials such as nanoparticles, pharmaceutical crystals, organic molecules and metal alloys.

We use grinding reactions to make ‘coordination polymers’, which are crystalline materials with an extended network held together by bonds between metal atoms and organic ligands. Many of these materials are porous, making them exciting candidates for gas storage, with one gram of the material having a surface area larger than a football pitch.
Our grinding method cuts out a lot of the energy-intensive and expensive processing needed to make coordination polymers. Typically coordination polymers are made using metal derivatives that have undergone a lot of processing from the ore to make reactive chemicals. However, in our process we were able to start from zinc oxide, which is a lot closer to the form in which the ore is dug out of the ground.
Because grinding reactions use little or no solvent, there is very little waste produced. This removes the environmental and financial costs of disposing of large amounts of solvent waste which is especially problematic on an industrial scale.
The mechanism of grinding reactions is not well understood. By shining a beam of X-rays though the reaction as it occurred, we were able to watch as the starting materials transformed, though various intermediate stages, into the products. We could then change our reaction conditions to understand the processes occurring. This new understanding will lead to cleaner, greener chemistry and help in the search for new materials.
References:
Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry
Real-time and in situ monitoring of mechanochemical milling reactions
NanoDTC PhD Student Cohort 2009
Links
www.fihm.msm.cam.ac.uk
http://www.chemistry.mcgill.ca/directory/people.php?p=1006&n=Fri%9Acic