Computer chips, industrial chemical reactions and detection of dangerous chemicals have all been improved by nanotechnology. Smaller parts packed closer and closer together have given us more sophisticated computer chips, more efficient ways of making medicines, and more sensitive chemical detectors. However, if we try to make an electronic device (e.g. a computer chip) with smaller parts, they behave differently, and they behave weirdly. Electric currents can jump across gaps they’re not supposed to, light can get trapped in gaps smaller than we expect and [insert other cool thing here]. Whenever we do this, thousands of scientists all around the world try to understand how these new systems work.
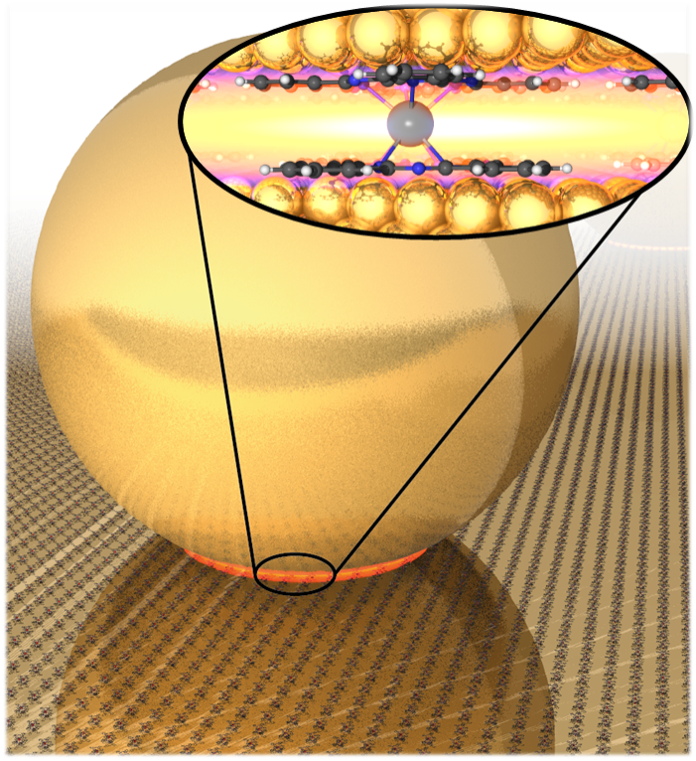
If we want to keep making better devices, we need a better understanding of how tiny solid objects interact at extremely short distances, and how we can control the position of these objects. To investigate this, we carefully assemble miniscule gold structures, put them under a microscope and fire lasers at them to see what colour they are. Using specially designed molecules as “glue”, we take gold particles, millions of times smaller than a grain of sand, and hold them less than a billionth of a metre apart. Changing the size of this gap by the tiniest fraction causes a huge shift in colour, one that nobody predicted.
Believe it or not, this wasn’t what we set out to do. We were trying to use our “specially designed” molecules to do chemical reactions. We haven’t figured out how to do that yet, but we accidentally discovered a totally new phenomenon! This happens a lot in science.
While we can’t do better chemical reactions at the moment, understanding this exciting new behaviour will give us insights into the behaviour of light itself. This understanding could be useful in the future for faster, molecule-based computers, specialised chemical sensors, or other useful things we haven’t thought of yet.